EN - Biorespira
Non-invasive ventilation for the COVID‑19 emergency
Doctors around the world agree that assisted lung ventilation is the most effective therapy in patients with COVID‑19 pneumonia, associated with ventilatory impairment. Many types of lung ventilators already exist on the market, but demand is higher than supply, with long lead times, at extremely high prices, and mostly designed for intensive care and intubated patients.
The World Health Organization (WHO) guidelines for the management of respiratory failure in COVID‑19 advocate the use of Non-Invasive Ventilation (NIV), provided that appropriate personal protective equipment is worn because the early intubation of a patient with known or suspected COVID‑19 and respiratory distress may be unnecessary, or their mechanical ventilation would have otherwise improved on NIV.*
*Reference: Arulkumaran N et al. Lancet Respir Med. 2020 Apr 20. pii: S2213-2600(20)30181-8.
Biorespira is designed from the ground up to respond to the COVID‑19 pandemic emergency
When there is no need for intubation
Non-invasive, portable solution
Usable in hospitals, nursing homes and homes
In case of low oxygen in the blood
Improves patient’s oxygenation
Isolated system prevents virus spread
To alleviate hospitals’ overload
Pre-hospitalization, to prevent the need for ICU treatment
Post-ICU treatment or hospitalization, to reduce the need for new intubation
For easy and immediate use
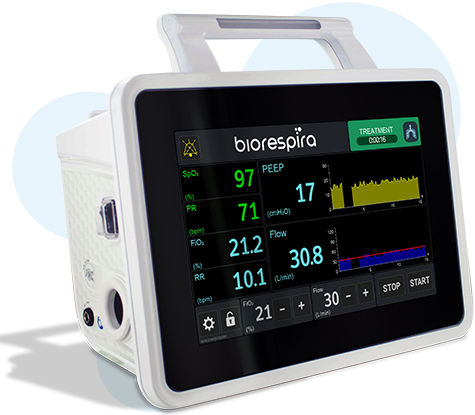
User-friendly touch screen interface
Cost-effective and innovative solution
Features
- High flow generator with a turbine capable of generating high flows starting from the ambient air, mixed with medical oxygen that can be supplied via an oxygen cylinder or medical gas plant
- Turbine operation optimizes oxygen consumption allowing the hospital's oxygen system to support more patients connected at the same time (one patient per Biorespira)
- Use with helmets and non-vented mask with antibaterial/antiviral filter creates an isolated system that prevents the risk of infection caused by the aerosol effect of the gas exhaled by the patient
- Direct measurement of PEEP pressure and integrated oximeter for a continuous reading of the actual air pressure delivered to the patient and oxygen saturation
- Allows for a humidifier, to be mounted on the inspiratory line
- Handle
- Weight 3 Kg (6.6 lb)
- Size 291 (W) x 259(H) x 203(D) mm
- 10" Display
- Touchscreen
- Direct reading of the PEEP, SpO2 parameters and respiratory rate (RR)
- 24V - switching power adapter AC/DC (100-240V 50-60Hz)
- Built-in patient bed coupling
- Oxygen hook-up through a cylinder or directly from the wall
- Integrated regulation and mixing of oxygen, adjustable from 21% to 100%
- High Flow Oxygen Therapy: Flow adjustable from 10 to 120 L/min (5L/min increments)
- PEEP pressure sensor
- Works with helmets, face masks and nasal cannulas. Use with helmets, non-vented masks, with PEEP valve and antiviral antibacterial filter creates an isolated system that prevents the risk of infection caused by the aerosol effect of the gases exhaled by the patient.
Biorespira can be used for:
- COVID-19 patients
- Pneumonia patients
- Patients with acute hypoxemic respiratory failure
- Patients needing pre-oxygenation before intubation
- Chronic obstructive pulmonary disease patients
- Post-intensive care patients
Technical Specifications
 Operation
Operation- temperature +18°C ÷ +40°C
- relative humidity 10% - 95%
- altitude 0-3000 m
 Trasport/Storage
Trasport/Storage- temperature -10°C ÷ +50°C
- relative humidity 10% - 95%, without condensation
 Dimensions
Dimensions291(W) x 259(H) x 203(D) mm
with built-in patient bed coupling
 Peso
Peso3 Kg (6.6 lb)
 Screen
ScreenTouchscreen - 10”
 Power supply
Power supply24V - switching adapter AC/DC (100-240V 50-60Hz)
 Degree of protection
Degree of protectionIP21
 Therapy
TherapyHFT e CPAP
 Compatible disposables
Compatible disposablesHelmet with PEEP valve
Non-vented face mask with PEEP valve
Nasal cannulas
Vented face mask*
*Require the use of a humidifer Patient Circuit
Patient CircuitMono-tube (adult)
 Filters
FiltersAntibacterial/Antiviral Filter
 Function mode
Function mode- Ventilation flux from 10 to 120 l/min
- O2 from 21% to 100%, compatible with oxygen cylinder or wall connections
- Patient’s PEEP pressure (cmH2O)
- Oxygen Saturation SpO2%
- Heart rate (bpm)
- Respiratory rate (bpm)
 Main characteristics
Main characteristics- Graphic display with therapy and control information
- Flow rate graph
- PEEP pressure graph
- Patient bed connection embedded
- Multi-Language
 Allarms
Allarmsaudible and visual
 Prestazioni pneumatiche
Prestazioni pneumatiche- O2
- Quick coupling for compressed oxygen
- Inlet Oxygen Gas pressure range of use 0.6 - 7 bar
- Air supply
- Integrated turbine with inlet air filter
- Inhale output for patient
- 22mm conical connector
 Classification
ClassificationClass IIa, hospital or domestic continuous function as per 93/42/CEE directive of the European Union Council
 Safety Classification
Safety Classification
Biorespira is Class IIa (Rule 11, Annex IX) with BF type applied part for continuous use
 Norme armonizzate
Norme armonizzateEN 60601-1/A12|EN 60601-1-8|IEC 60601-1-11|EN 60601-1-6|IEC 62366-1|EN 62304/AC|ISO 10993-1
 Compatibilità elettromagnetica
Compatibilità elettromagneticaComplies with the requirements of EN 60601-1-2
 Declaration
DeclarationThe Biorespira ventilator has been developed to conform to the applicable international standards and the established guidelines. The ventilator is manufactured following the EN ISO 13485, EN ISO9001 standards and the 93/42/CEE directive of the European Union Council, Annex II, Article 3 related to the assurance of certified quality. The ventilator conforms to the essential requirements of the 93/42/CEE directive of the Council
Contact us to learn more about Biorespira
Please provide the following information and we will get back to you quickly with more information on your request.